Apply for access
Preparing to apply
If you have already registered with UK Biobank, you can apply to access the database via the Access Management System (AMS). To complete an application form you will need the following information:
- A summary of the research you intend to conduct
- The UK Biobank data-fields you require
- A description of any new data or variables your research will generate
How to complete an access application
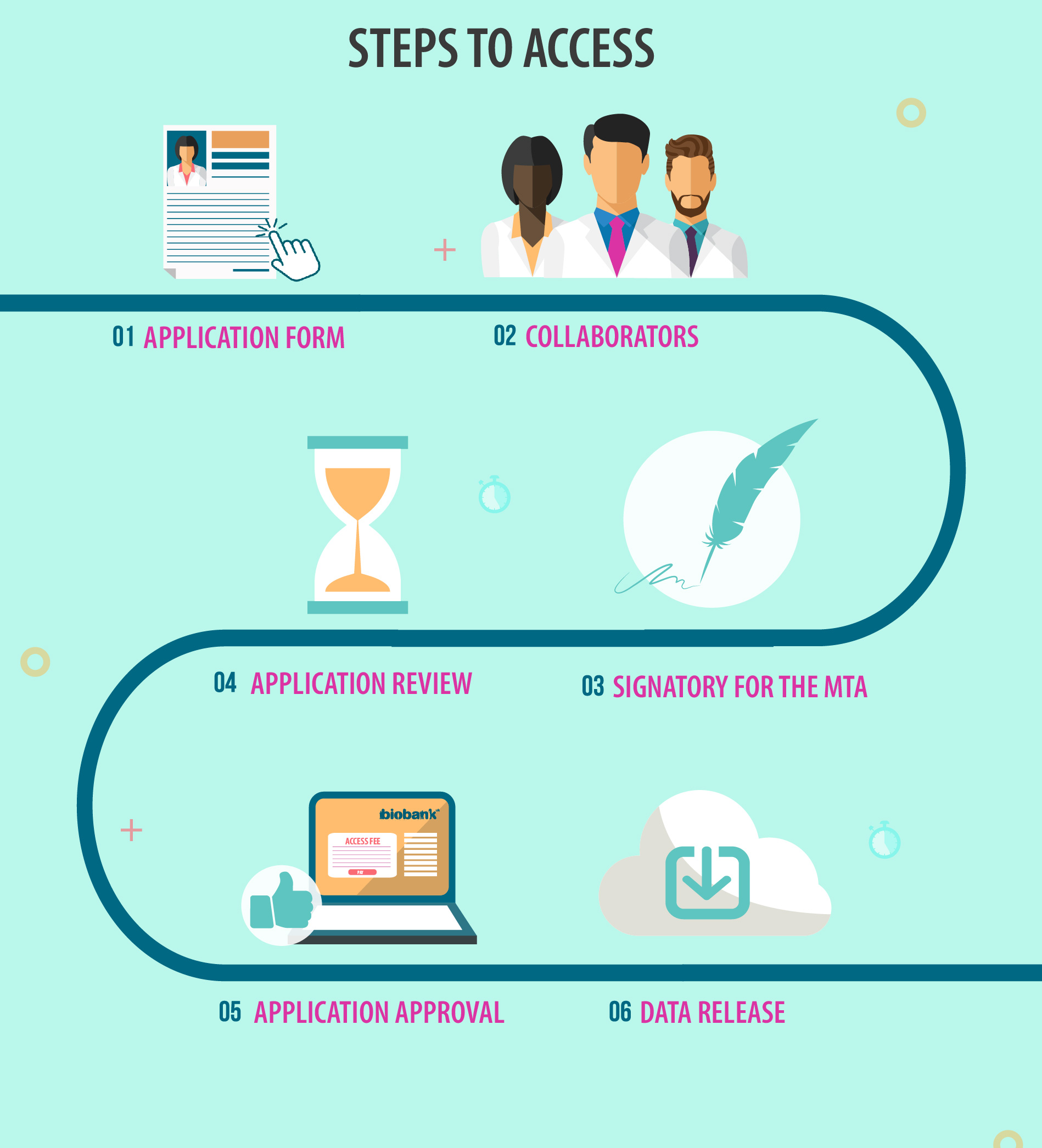
Step 1: Complete the application form in the UK Biobank online Access Management System (AMS) and select a data tier.
Step 2: Add researchers who will access UK Biobank data to the application as collaborators.
Step 3: Add contact information for a signatory who is authorised to sign the material transfer agreement (MTA) on behalf of your institute. This must be someone other than the PI/Lead Collaborator for each institute.
Step 4: The UK Biobank team will review your application and contact you via a message in the AMS with any feedback.
Step 5: On approval of your application, an access fee request will be raised, and the MTA will be sent to you and your authorised signatory.
Step 6: Following payment of the access fee and return of signed MTA, the Access team will inform you when your data have been released and are available to access.
For more details on the application process, please view:
The vetting process
Trained personnel carry out background checks on any researcher who applies for access. They review the researcher's professional history and look for evidence that they have carried out high-quality health-related research. They must be working for a legitimate research organisation with a track record of health-related research. All researchers are checked against international sanctions lists.
Our in-house scientists then assess whether the research proposal qualifies as health-related research in the public interest. This means the findings should be likely to benefit the health and wellbeing of society, and should not cause harm, such as perpetuate stereotypes about certain groups.
If there is any doubt about this, or if the proposal raises any concerns, it is referred to UK Biobank's expert Access Committee which will consider the application in more detail and seek ethics advice if needed.
All applications go through this process, regardless of whether the researcher works for a university, a charity or a company. UK Biobank has approved more 4500 applications, and rejected many others.
Up until January 2025, we would consider applications from insurance companies; for example, if assessing the risk of disease to reduce both over and under-estimates. However, our Access Committee has reviewed this policy, in consultation with our Participant Advisory Group and Ethics Advisory Committee, and concluded:
- It will no longer approve applications from insurance companies for direct access to de-identified UK Biobank data;
- Some approved researchers may work with, or be funded by, insurance companies; for example, if they are seeking to understand the rate at which a disease develops. This does not grant the insurance company access to participant data;
- Insurance companies may use or reference publicly-available findings published in peer-reviewed journals by approved researchers.
Are you in a hurry?
Tips to minimise the application timeline:
- Ensure your legal team has seen our standard MTA at the point of applying (please note, our MTA is non-negotiable)
- Amend your application in response to our feedback as soon as you can – keep an eye out for email alerts saying you have a message from us
- Pay the access fee online via the AMS
- Sign the MTA we send to you and your authorised signatory (via DocuSign) as soon as you can.

Follow our easy tips to speed up your application
A note about your lay summary
You will be asked to provide a short summary of your work in lay language. This is important because it allows participants and members of the public to understand how UK Biobank is being used. The lay summary should reflect all aspects of your work and match with your publications. It should indicate whether or not findings may be considered as controversial.
Cost
The fee to access UK Biobank helps cover the project's running costs and there are some circumstances in which UK Biobank will offer financial support. Click on the button below to find out more.
Making payments
Payment can be made via our online payment system in AMS or via BACS transfer from your institute. If making payment via BACS transfer, you will need to generate invoice in AMS (see the AMS User Guide for instructions). All payments must be made in GBP.
Please note that UK Biobank’s policy is not to complete supplier forms. Please see the FAQ below for details.
Biological samples
UK Biobank’s policy for the assay of depletable biological samples is that the sample should be efficiently turned into non-depletable data.
If you are submitting an application to request biological samples please ensure your application meets our Sample Release Policy, then complete and return a PMG103_B form to the Access Management Team.
Re-contact
If you wish to re-contact participants, you will need to provide additional information to justify this request. Applications are reviewed in line with the UK Biobank’s policy to uphold participant goodwill and trust in the resource. Full details can be found in our 'Re-Contact Procedures'.
All requests to re-contact UK Biobank participants, and for biological samples, are considered by the UK Biobank Access Sub-committee, which meets quarterly.
A note on costs and capacity
Projects requiring access to samples are individually costed once all the details are confirmed.
Typically, it costs in the region of £5 to £10 (excl. VAT) per sample for UK Biobank to supply biological samples for our entire cohort. There are many factors that can influence the cost per sample and consequently it is difficult to provide accurate estimates before the project is fully reviewed. For example, cost can be impacted by the number of samples, how they might be selected for supply and dispersed through our archives and storage technologies, the timescale of the project, required labware and complexity of data processing of the results.
UK Biobank are undertaking a full replacement of our infrastructure, which limits capacity to deliver samples for the next few years. After 2026, capacity should be significantly increased and the cost of sample supply will be reviewed.
Share your research experience
We are always eager to showcase researchers that have used UK Biobank's biomedical database and research resource to make scientific discoveries that improve human health. If you are happy to be featured in a case study or other material, click on the button below to email the communications team.
Last updated
